Purpose-Built eLearning for Third Parties
Heighten third party awareness and elevate standards with a suite of risk-specific online courses and refreshers
Interactive eLearning for third parties
Online multi-lingual compliance training can help your business to quickly and effectively raise awareness amongst third parties on how to identify business risks in real time while also shielding themselves, and your organization, from legal and reputational damage. Our purpose-built courses are available via our own learning management system (LMS) embedded within the Ethixbase360 Third Party Risk Management platform or can be deployed on your own LMS.
Curated Multi-lingual Courses
Access our library of over 15 curated courses that address key third party risk areas. Courses can be made available to third parties in up to 34 languages depending on the module. Topics include global anti-bribery compliance, forced labor and human trafficking, conflicts of interest, gifts and hospitality, global anti-trust and competition law and more. Users have access to engaging course content that varies in length and interactivity options including interactive videos, infographics and gaming components for best learner absorption.
Seamless User Registration
Courses can be allocated to third parties automatically as part of a risk-based workflow or manually via the Ethixbase360 TPRM platform. Once allocated, third parties can disseminate courses to multiple-learners throughout their organization. Certificates are issued seamlessly to candidates upon successful completion.
Fully Integrated
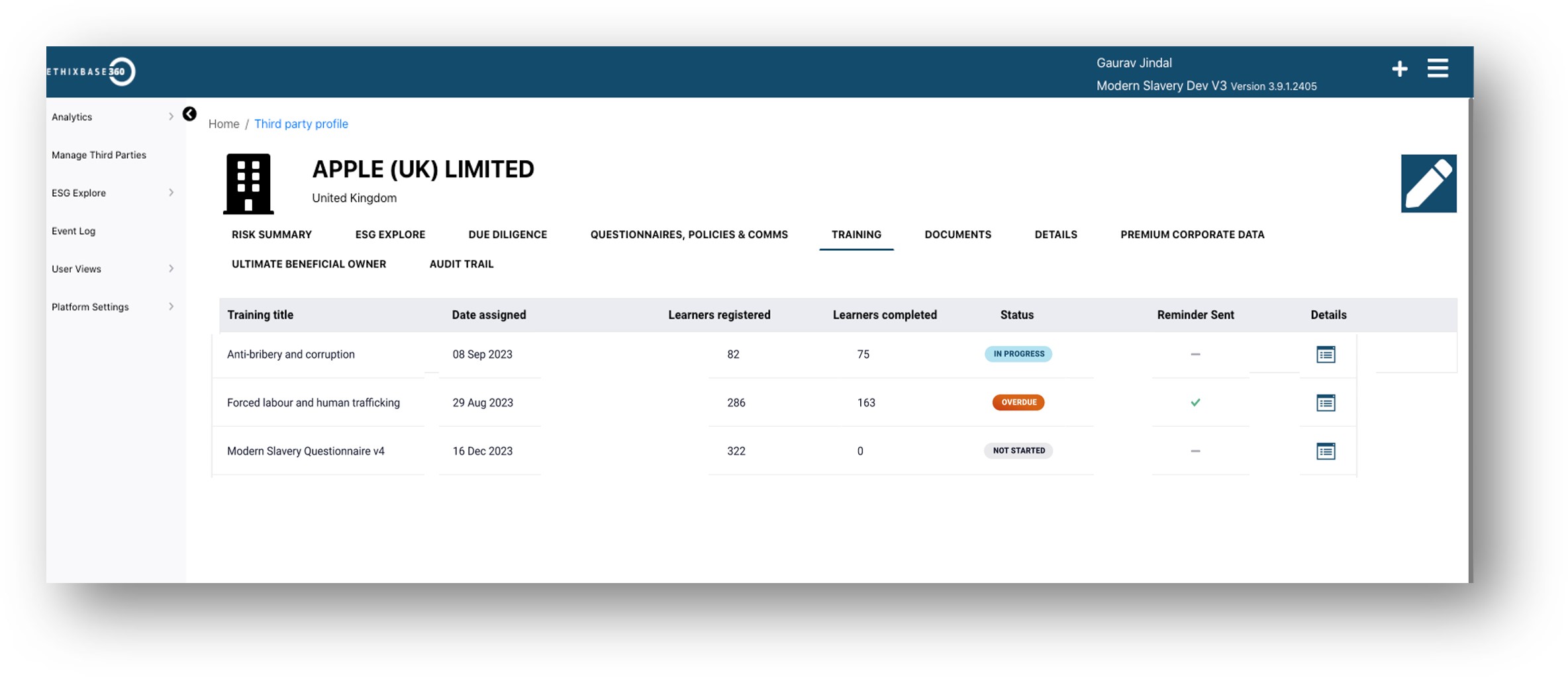
eLearning is fully embedded within the Ethixbase360 Third Party Risk Management platform for full reporting on course status, completion and results to support training compliance and audit records.
Training is an essential element of an effective compliance program, and it is increasingly important and expected for companies to train their third parties on key risk areas to mitigate potential risks and evidence their approach.
Our Content or Yours. Your LMS or Ours
Roll out our off the shelf courses, customize these based on your business's specific needs or work with us to digitize custom courses for your third parties based off existing in-house materials.
Our eLearning modules are SCORM compliant and can be administered either via our state-of-the-art Learning Management System (LMS) or via your own LMS if required.

Engage with third parties directly with easy-to-complete, responsive questionnaires with market leading response rates.
Empower third parties to own their own compliance credentials and facilitate rapid information exchange.
Get the most out of your TPRM program by leveraging experienced analysts to deploy direct personalized engagement with third parties.
Explore, Enhance, Engage
Safeguard your reputation with a 360° view. Try Ethixbase360 now.
Resources
Our Latest News, Case Studies, Webinars & More

The First FCPA Resolution After the Enforcement Pause and What It Signals For Risk Management
On November 10, 2025, Comunicaciones Celulares S.A. (“Comcel”), a subsidiary of Millicom International Cellular, entered into a two-year deferred prosecution agreement with the U.S.[...]
Statement from Our CEO Simon Wardle on International Anti-Corruption Day 2025
#UnitedAgainstCorruption | #IACD2025 On International Anti-Corruption Day, we stand with partners around the world #UnitedAgainstCorruption.[...]
Five Key Takeaways from “Managing Cyber Risk in Your Supply Chain”
17 November 2025 — Ethixbase360, a global leader in third-party risk management and supply chain transparency solutions, and Veresure, a technology-driven integrity and risk-management company based in Australia that helps organisations[...]
Ethixbase360 and Veresure Announce Strategic Partnership to Strengthen Third-Party Risk Management and Supply Chain Integrity Amid Growing Global Compliance Demands
17 November 2025 — Ethixbase360, a global leader in third-party risk management and supply chain transparency solutions,[...]
Human Rights Due Diligence in the Asia Pacific Region: The Path From Voluntary to Mandatory
Human rights due diligence (HRDD) is rapidly gaining ground across the Asia Pacific (APAC), as governments have taken steps to […]