Third-Party Risk Management for Life Sciences
Ethixbase360 smart automation with expert-led due diligence and responsive support gives our clients a clear, connected view of global third-party risk to withstand regulatory scrutiny and address complex global value chains with speed, flexibility and responsiveness.
Driving transparency, compliance, and resilience across your global third-party ecosystem.
The Ethixbase360 third-party risk management platform brings together intelligent automation, expert-led due diligence, and responsive support — giving Life Sciences organizations a unified global view of third-party risk to meet evolving regulatory demands and manage complex value chains with speed and agility.
Pressures of Life Sciences Today
Escalating Regulatory Demands
With global compliance standards evolving across anti-bribery, sanctions, and ethical sourcing, risk teams must adapt quickly to avoid costly enforcement actions, product disruptions, and reputational harm.
Complex Global Value Chains
Life Sciences organizations rely on vast, interconnected supplier networks. Without centralized oversight, siloed local approaches create blind spots and increase exposure to hidden risks.
Reputational Vulnerability
Even a single third-party misstep can result in public scrutiny and reputational harm. In Life Sciences, trust is everything and it can be lost in an instant.
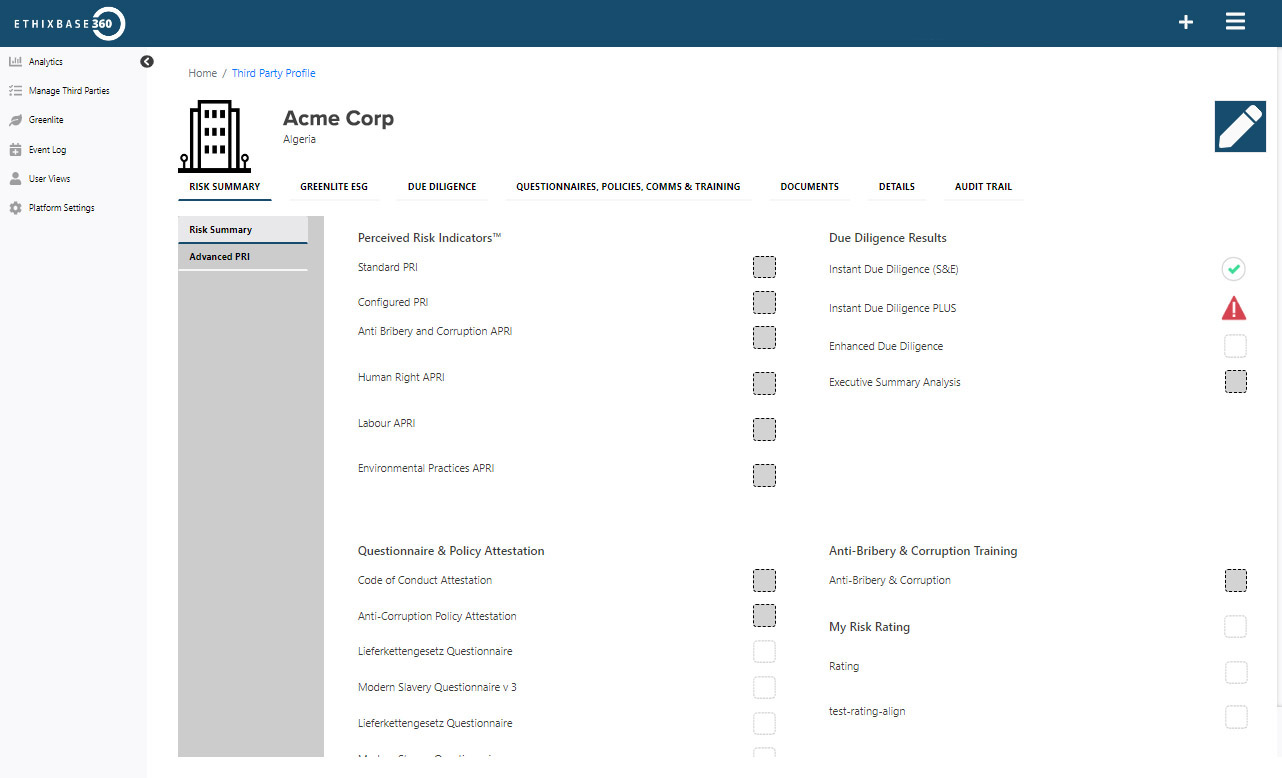
Integrated risk intelligence
Gain a connected, real-time view of third-party risk, enabling you to act quickly, meet regulatory demands.
- Rapid onboarding with built-in pre-screening workflows
- Instant entity verification and alerts
- Risk-based screening, escalation, and investigation, all in one platform.
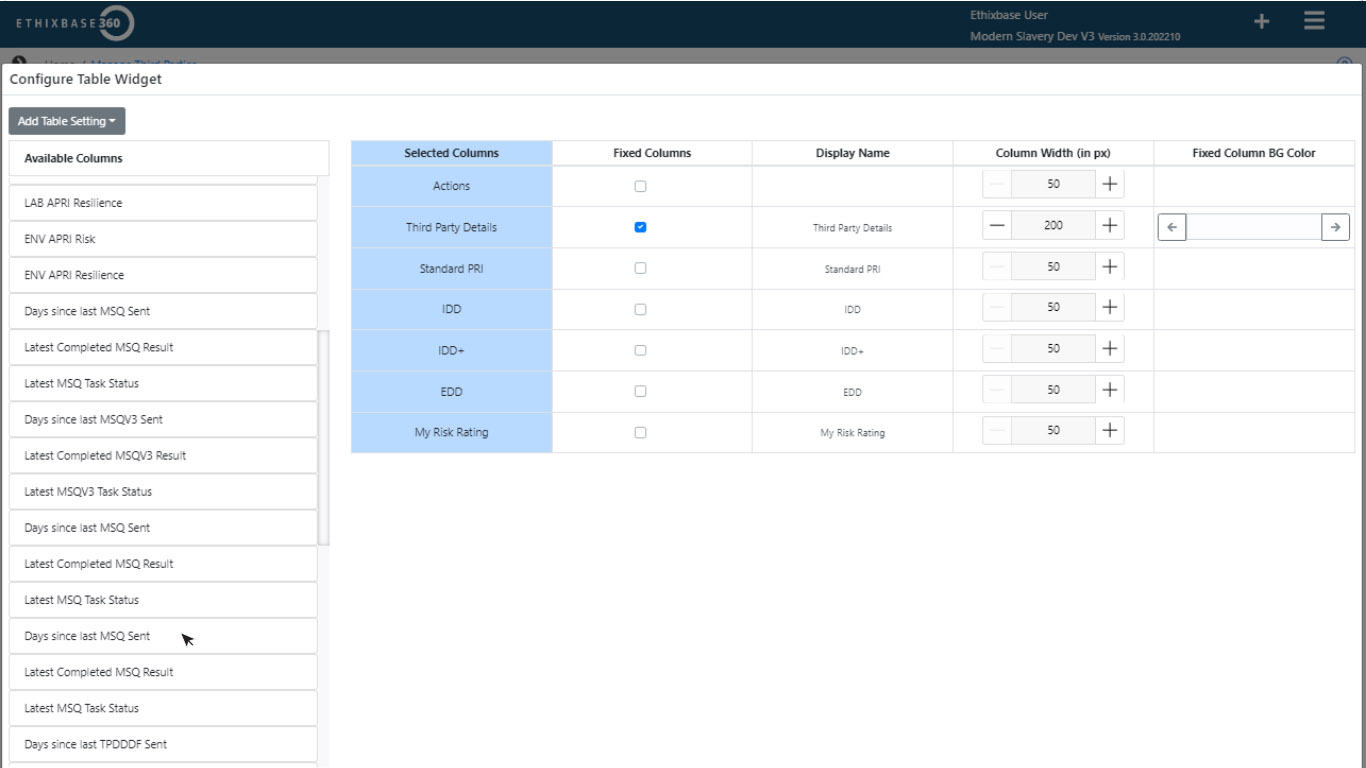
Compliance at the heart of operations
Demonstrate compliance across global regulatory frameworks while creating a transparent, defensible compliance culture.
- Configurable Enhanced Due Diligence tailored to your needs
- Seamless vendor assessments (e.g., anti-bribery, modern slavery)
- Policy distribution and confirmation tracking for third-party accountability.
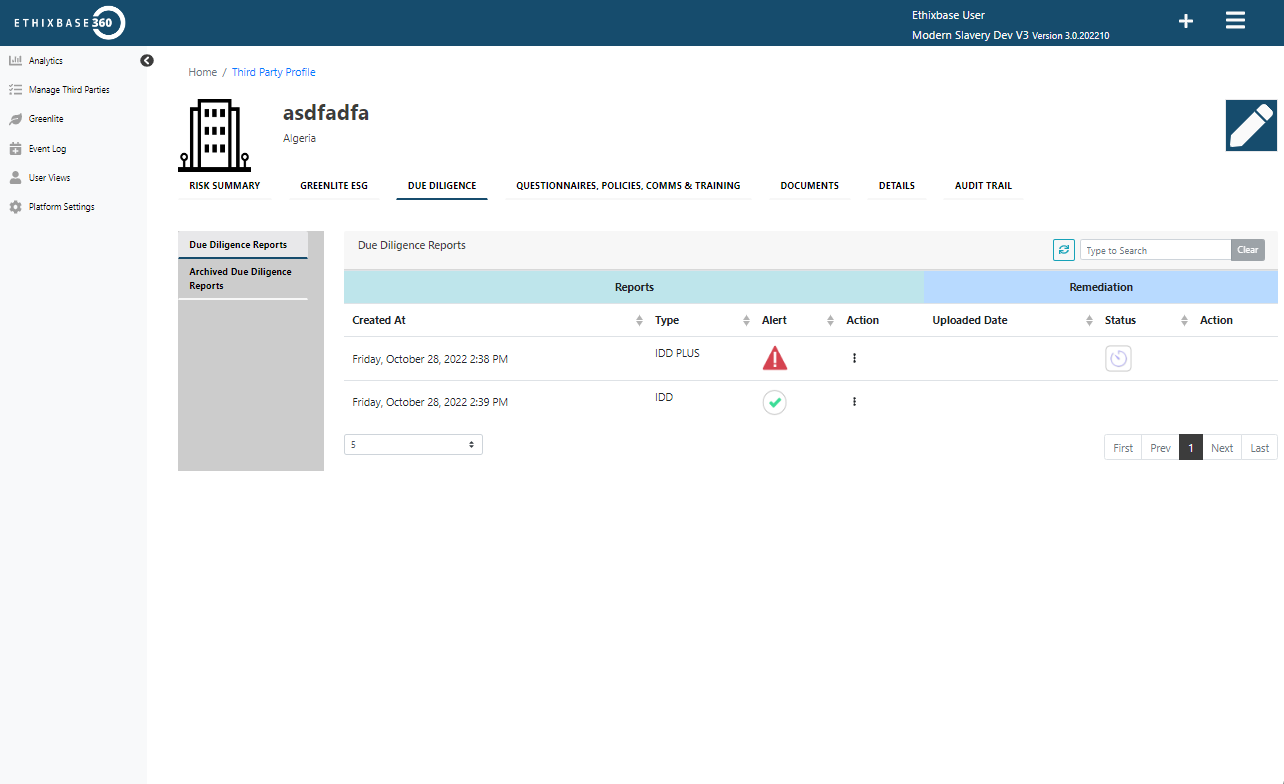
A platform built around you
Drive adoption across the enterprise, eliminate silos, and scale oversight efficiently across global operations.
- SSO, API connectivity, and reporting integrations
- Automated approvals, questionnaires, and case management
- Standardized reporting and business intelligence for faster decisions.










Ready to Transform Your
Third-Party Risk Management?
Discover how Ethixbase360 can help your organization build a transparent, compliant, and resilient third-party ecosystem.
“Everything is valuable in Ethixbase360’s platform, from the moment they trigger the automatic email directly to the third parties, the third parties respond directly via the platform, the Enhanced Due Diligence (EDD) reports are good, the Instant Due Diligence Plus (IDD+) is a very good complement for changes in management. The whole process is very useful.”
Head Group of Compliance
Fortune 500 Pharmaceutical Company
“Ethixbase360 has been really helpful and responsive. The platform itself is very user-friendly and everything now is much more organized for us and there’s a lot more automation in the process now. They have been really helpful with not just the product, but also suggestions. They’ve sometimes steered us towards more cost-effective things that would provide a similar level of protection. I’ve never been frustrated with waiting for a response.”
Group Compliance Manager, Global Medical Devices Suppliers
Resources
Our Latest News, Case Studies, Webinars & More

The First FCPA Resolution After the Enforcement Pause and What It Signals For Risk Management
On November 10, 2025, Comunicaciones Celulares S.A. (“Comcel”), a subsidiary of Millicom International Cellular, entered into a two-year deferred prosecution agreement with the U.S.[...]
Statement from Our CEO Simon Wardle on International Anti-Corruption Day 2025
#UnitedAgainstCorruption | #IACD2025 On International Anti-Corruption Day, we stand with partners around the world #UnitedAgainstCorruption.[...]
Five Key Takeaways from “Managing Cyber Risk in Your Supply Chain”
17 November 2025 — Ethixbase360, a global leader in third-party risk management and supply chain transparency solutions, and Veresure, a technology-driven integrity and risk-management company based in Australia that helps organisations[...]
Ethixbase360 and Veresure Announce Strategic Partnership to Strengthen Third-Party Risk Management and Supply Chain Integrity Amid Growing Global Compliance Demands
17 November 2025 — Ethixbase360, a global leader in third-party risk management and supply chain transparency solutions,[...]
Human Rights Due Diligence in the Asia Pacific Region: The Path From Voluntary to Mandatory
Human rights due diligence (HRDD) is rapidly gaining ground across the Asia Pacific (APAC), as governments have taken steps to […]